It took a really long time for this document. First, several years for its preparation and then for its publication, because this did not happen at all immediately after its adoption by the government. It took more than a month, during which many questions were asked about the document.
The resolution on the National Plan came into force on the day it was adopted, i.e. 24 August, but it was not published in the Polish Monitor until 27 September.
-The document is the result of years of work by teams appointed by the Minister of Health and at the same time implements the recommendation of the EU Council to develop a national strategy for rare diseases. Although the Rare Disease Plan is not a legal act, but only an operational one, it plays an important role in defining the desired directions for changes in the care of patients suffering from rare diseases. It defines six areas for which an analysis of the current state has been carried out and creates a list of necessary actions to be taken, together with an indication of the modalities and timing of their introduction and the model for financing their implementation, explains Marcin Pieklak, partner at DZP.
The implementation of the Plan is set for the period 2021-2023.
The document aims to improve the quality of medical care for patients with rare diseases.
The plan includes:
1) criteria for appointment and operation Centres of Expertise on Rare Diseases responsible for overseeing the diagnosis, diagnosis and treatment of patients with rare diseases;
2) Identify directions for improving the diagnosis of rare diseases, including access to modern diagnostics using genomic technologies;
3) proposals to improve access to medicines, medical devices and foodstuffs for special nutritional uses for rare diseases;
4) launching a monitoring system for rare diseases by setting up a Polish Register of Rare Diseases;
5) the creation of individual medical recordswhich will include clinical data - Rare Disease Patient Passport;
6) the creation of a "Rare Diseases" Information Platform, containing clinical, scientific and organisational information on rare diseases.
Access to medicines and multi-criteria analysis
The main aim of the proposed changes to reimbursement is to improve access to medicines, medical devices and foodstuffs for particular nutritional uses used in rare diseases.
-Recall that one of the tasks identified by the Plan is: to carry out an analysis towards the introduction of multi-criteria decision-making analysis and to define and introduce in the reimbursement law the amount of the threshold for the cost of an additional quality-adjusted life year for technologies with approval for rare diseases, recalls counsel Pieklak.
-The idea of introducing a new tool in the form of a multi-criteria analysis for the assessment of technologies used in rare diseases has also appeared in the National Drug Policy for 2018 - 2022. The Plan for Rare Diseases indicates possible approaches to the implementation of multi-criteria analysis into the Polish drug technology assessment system, which can help to develop an appropriate model, says Kinga Frelas of DZP.
- Many of the tasks formulated with regard to the availability of therapies reflect demands that have been made in the public space for many years. Therefore, it seems justified to take measures aimed at their fastest possible implementation. The above actions are also supported by the legislative changes planned by the Polish legislator, including the Reimbursement Act. Taking into account that we have been waiting for many years for solutions dedicated to orphan therapies, it can be expected that the relevant authorities will take measures aimed at their rapid implementation - recalls Pieklak.
Other amendments to the Reimbursement Act
The entry into force of the national plan will involve an amendment to the Reimbursement Act - which is expected to take place by the end of this year. It will add, among other things:
"(30) rare diseases - diseases occurring with a prevalence of less than 5 per 10 000 persons in the population;
31) medicines intended for therapies for rare diseases - medicines dedicated to the treatment of diseases occurring at a frequency of less than 5 per 10 000 persons in the population, irrespective of their designation under separate legislation as orphan medicinal products."
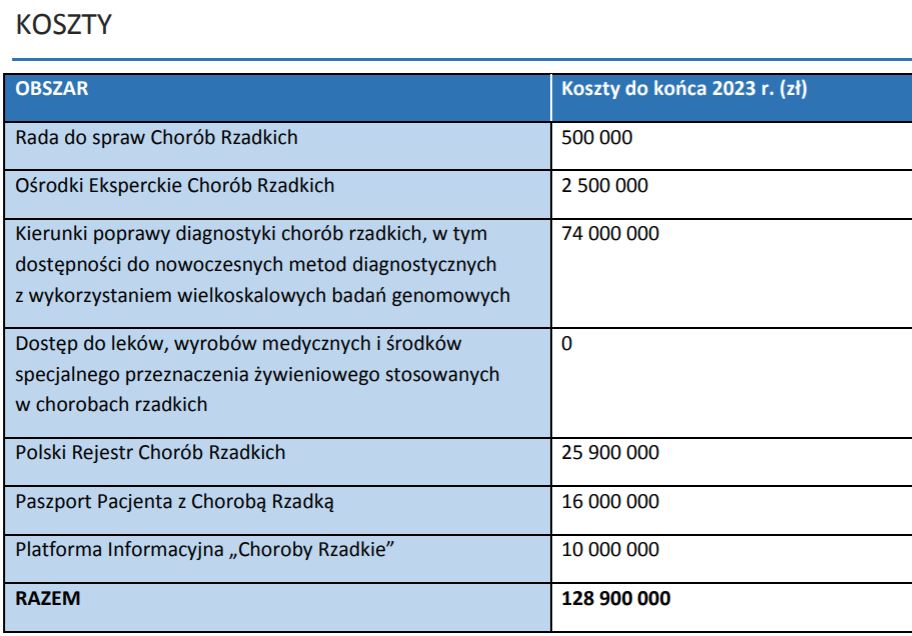
Expenditure
Expenditure on implementation is expected to be close to £130m, but not annually, but for the entire plan until the end of 2023.
See the full document: Resolution No. 110 of the Council of Ministers of 24 August 2021 on the adoption of the document Plan for Rare Diseases
Source: cowzdrowiu.pl